Researchers from Penn Dental and Penn Engineering have developed a new way to rapidly and precisely eradicate fungal infections in the mouth by using nanorobots guided by magnets.
Original Article by Nathi Magubane
Infections caused by fungi, such as Candida albicans, pose a significant global health risk due to their resistance to existing treatments, so much so that the World Health Organization has highlighted this as a priority issue.
Although nanomaterials show promise as antifungal agents, current iterations lack the potency and specificity needed for quick and targeted treatment, leading to prolonged treatment times and potential off-target effects and drug resistance.
Now, in a groundbreaking development with far-reaching implications for global health, a team of researchers jointly led by Hyun (Michel) Koo of the University of Pennsylvania School of Dental Medicine and Edward Steager of Penn’s School of Engineering and Applied Science has created a microrobotic system capable of rapid, targeted elimination of fungal pathogens.
“Candida forms tenacious biofilm infections that are particularly hard to treat,” Koo says. “Current antifungal therapies lack the potency and specificity required to quickly and effectively eliminate these pathogens, so this collaboration draws from our clinical knowledge and combines Ed’s team and their robotic expertise to offer a new approach.”
The team of researchers is a part of Penn Dental’s Center for Innovation & Precision Dentistry, an initiative that leverages engineering and computational approaches to uncover new knowledge for disease mitigation and advance oral and craniofacial health care innovation.
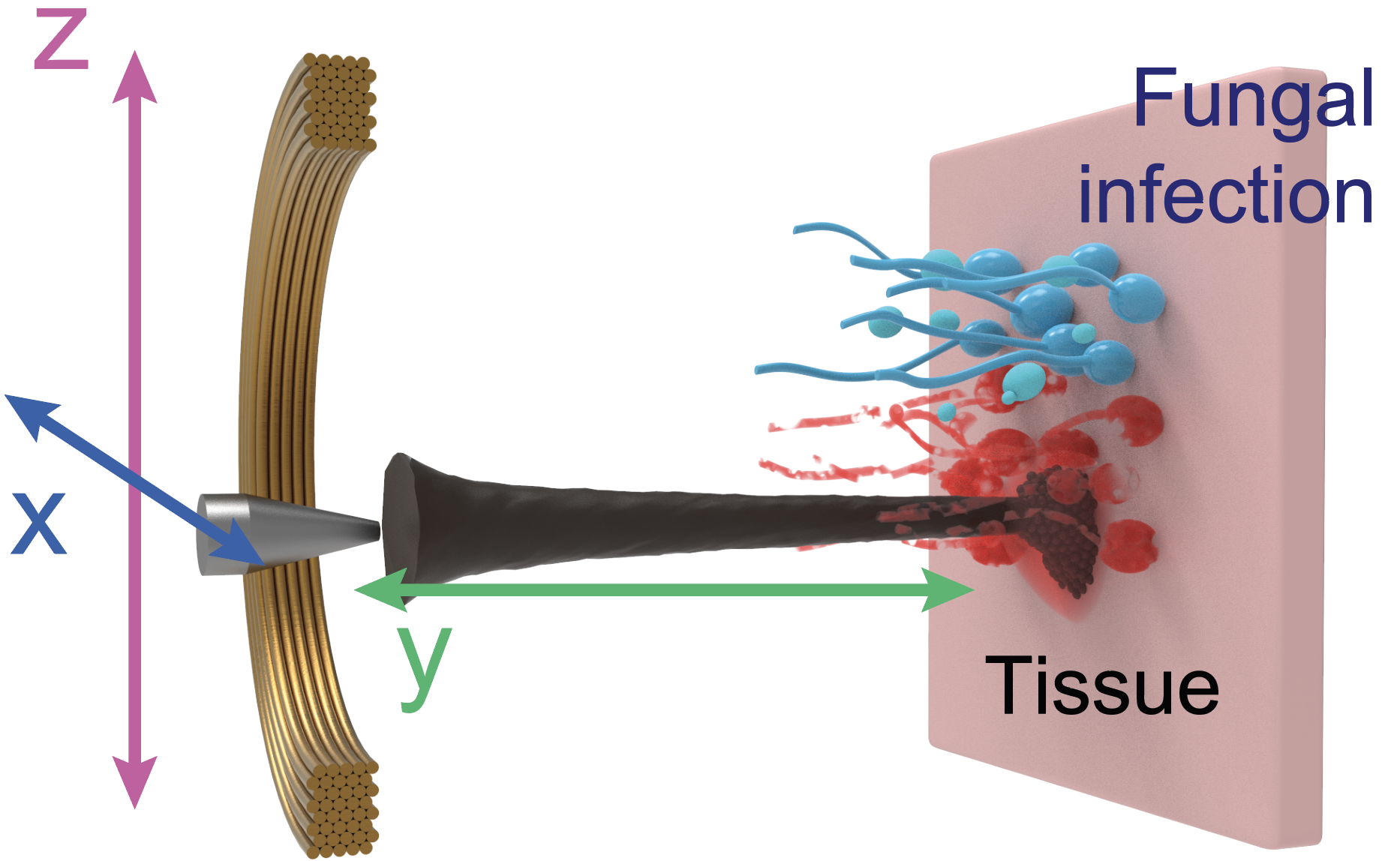
For this paper, published in Advanced Materials, the researchers capitalized on recent advancements in catalytic nanoparticles, known as nanozymes, and they built miniature robotic systems that could accurately target and quickly destroy fungal cells. They achieved this by using electromagnetic fields to control the shape and movements of these nanozyme microrobots with great precision.
“The methods we use to control the nanoparticles in this study are magnetic, which allows us to direct them to the exact infection location,” Steager says. “We use iron oxide nanoparticles, which have another important property, namely that they’re catalytic.”
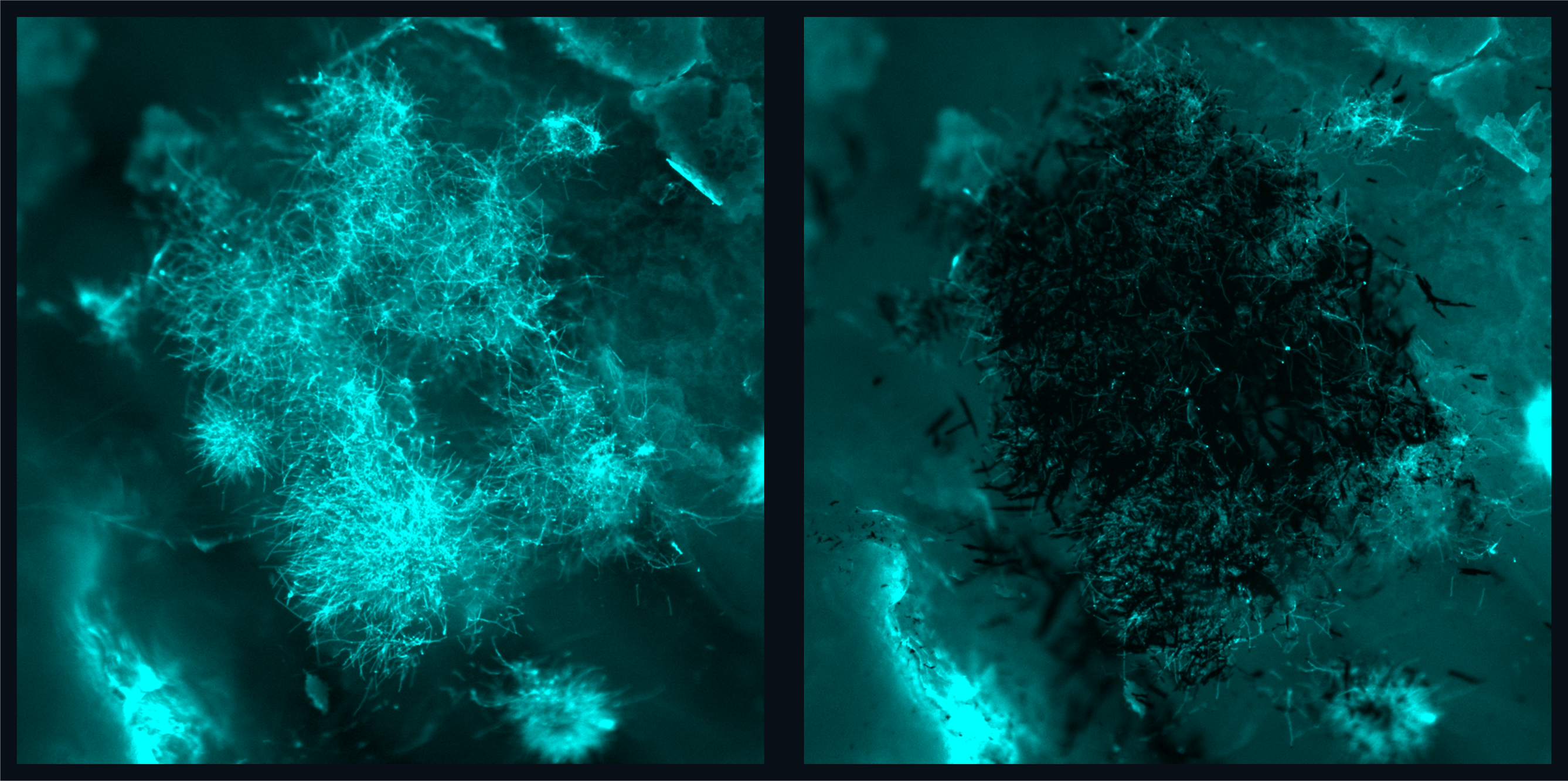
Steager’s team developed the motion, velocity, and formations of nanozymes, which resulted in enhanced catalytic activity, much like the enzyme peroxidase, which helps break down hydrogen peroxide into water and oxygen. This directly allows the generation of high amounts of reactive oxygen species (ROS), compounds that have proven biofilm-destroying properties, at the site of infection.
However, the truly pioneering element of these nanozyme assemblies was an unexpected discovery: their strong binding affinity to fungal cells. This feature enables a localized accumulation of nanozymes precisely where the fungi reside and, consequently, targeted ROS generation.
“Our nanozyme assemblies show an incredible attraction to fungal cells, particularly when compared to human cells,” Steager says. “This specific binding interaction paves the way for a potent and concentrated antifungal effect without affecting other uninfected areas.”
Coupled with the nanozyme’s inherent maneuverability, this results in a potent antifungal effect, demonstrating the rapid eradication of fungal cells within an unprecedented 10-minute window.
Looking forward, the team sees the potential of this unique nanozyme-based robotics approach, as they incorporate new methods to automate control and delivery of nanozymes. The promise it holds for antifungal therapy is just the beginning. Its precise targeting, rapid action suggest potential for treating other types of stubborn infections.
“We’ve uncovered a powerful tool in the fight against pathogenic fungal infections,” Koo says. “What we have achieved here is a significant leap forward, but it’s also just the first step. The magnetic and catalytic properties combined with unexpected binding specificity to fungi open exciting opportunities for an automated ‘target-bind-and-kill’ antifungal mechanism. We are eager to delve deeper and unlock its full potential.”
This robotics approach opens up a new frontier in the fight against fungal infections and marks a pivotal point in antifungal therapy. With a new tool in their arsenal, medical and dental professionals are closer than ever to effectively combating these difficult pathogens.
Hyun (Michel) Koo is a professor in the Department of Orthodontics and in the divisions of Pediatric Dentistry and Community Oral Health and is the co-founder of the Center for Innovation & Precision Dentistry in the School of Dental Medicine at the University of Pennsylvania.
Edward Steager is a research investigator in the School of Engineering and Applied Science’s General Robotics, Automation, Sensing & Perception Laboratory at Penn.
Other authors include Min Jun Oh, Alaa Babeer, Yuan Liu, Zhi Ren, Zhenting Xiang, Yilan Miao, and Chider Chen of Penn Dental; and David P. Cormode and Seokyoung Yoon of the Perelman School of Medicine.
This research was supported in part by the National Institute for Dental and Craniofacial Research (R01 DE025848, R56 DE029985, R90DE031532 and; the Basic Science Research Program through the National Research Foundation of Korea of the Ministry of Education (NRF-2021R1A6A3A03044553).
